
Elon Musk’s medical technology company, Neuralink, has achieved a major breakthrough by reportedly implanting a brain chip into a human subject. While this marks a significant milestone, the development is not without controversy, with ongoing investigations into potential violations of animal welfare laws and concerns about the rushed timelines. This article explores the details of Neuralink’s human trial, the intended benefits of the brain chip, and the ethical considerations surrounding the technology.
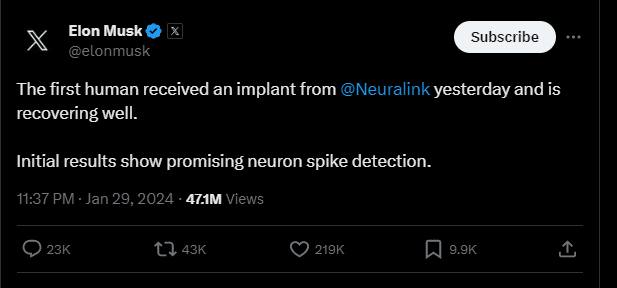
Elon Musk’s Announcement: Elon Musk took to Twitter to announce that Neuralink has successfully implanted a brain chip into its first human subject. The CEO expressed optimism about the initial results, stating that the patient is recovering well, and promising neuron spike detection has been observed.
Volunteer Criteria: Neuralink’s call for trial participants specified criteria such as being within the U.S., over 18, and having a disability. The company is particularly interested in individuals with conditions like quadriplegia, paraplegia, vision or hearing loss, and major limb amputation. The first trial targeted those with quadriplegia due to spinal cord injury or amyotrophic lateral sclerosis (ALS).
Telepathy – Neuralink’s First Product: Musk revealed that Neuralink’s initial product is named Telepathy, designed to enable disabled individuals to operate electronic devices using their brain. The goal is to empower those who have lost the use of their limbs to control devices by simply thinking.
PRIME Study: Neuralink’s Precise Robotically Implanted Brain-Computer Interface (PRIME) Study aims to assess the safety of the N1 brain implant and the R1 surgical robot used for implantation. The N1 Implant’s flexible threads are intended to record and transmit brain signals wirelessly, with the initial goal of allowing users to control a computer cursor or keyboard through thoughts.
Controversies and Investigations: Neuralink has faced controversies, including investigations into potential violations of animal welfare laws. Allegations suggest that rushed timelines led to botched experiments and unnecessary suffering in animals. The company received FDA approval for human trials in May, raising concerns about the careful handling of human subjects.
Ethical Considerations: Despite the potential benefits for individuals with disabilities, there are ethical considerations surrounding the implantation of brain chips. The article encourages potential candidates to carefully evaluate the risks and benefits, given Neuralink’s history and the delicate nature of brain-related interventions.
Neuralink’s successful implantation of a brain chip in a human marks a significant step toward developing technology that could benefit individuals with disabilities. However, ongoing controversies, investigations, and ethical considerations underscore the need for cautious progress. As Neuralink advances its technology, transparency, ethical practices, and the well-being of both animal and human subjects remain critical aspects of its journey.
Related posts:
 Leaked Google Database Reveals Significant Privacy Breaches Affecting Various User Groups
Leaked Google Database Reveals Significant Privacy Breaches Affecting Various User Groups
 Apple Unveils Upgraded MacBook Air Models: M3 Chip Takes Center Stage
Apple Unveils Upgraded MacBook Air Models: M3 Chip Takes Center Stage
 Apple 2024 MacBook Air 15-inch with M3 Chip: The Ultimate Portable Powerhouse
Apple 2024 MacBook Air 15-inch with M3 Chip: The Ultimate Portable Powerhouse
 Apple 2023 iMac All-in-One Desktop Computer Review: Supercharged with the M3 Chip
Apple 2023 iMac All-in-One Desktop Computer Review: Supercharged with the M3 Chip



Leave a Comment
You must be logged in to post a comment